
This is my full setup with the 3 gallon still, my StoveTec Rocket Stove, and both a collection bucket and cooling water bucket.
Water Jacket Moonshine Still
On Ebay:https://www.ebay.com/usr/chanceair
All “moonshine stills” on Ebay (including coil type)
You know the funniest thing? When someone posts a comment on one of these articles that starts with “You can just..”. One hundred percent of the time it is someone who read a tip on a survival board or something and they are here to parrot an idea that may or may not be true. But what I have found in working on this column, every single time, is that while knowledge may be power, experience is survival. My experience with distilling water was not easy using fuel and means that would be available in a post collapse world. Oh my goodness it is slow going, about 1/2 gallon an hour using the distiller yous see here. “You can just…” distill dirty water was a comment that I got very early on in this series, and it just isn’t as easy as you would think it would be. Check out the pictures below, using a 3 gallon still from the Ebay seller linked above, and my Rocket Stove. I did get good, clean, drinkable water from yellow sulfur water, but it was not fun.

The water that I used for this project came from my pond which was dug earlier this year. It is horribly full of sulfur and undrinkable.
Water is of course the core of any survival plan. You can live for weeks without food, but without water you’ll be gone in a couple of days generally. Your water has to be clean. Bad water kills you quicker and meaner than no water, and water can have any number of potentially deadly pathogens and chemicals. You can filter your water, and I covered a number of good filters in the early days of this series, but eventually filters clog and no longer work. If you can’t replace filters, and all you have is dirty, dangerous water, you may have to think about distillation.
To start, not all water has to be distilled just to be drinkable. Most harmful bacteria, cysts and all viruses die long before water even gets to boiling, and if you boil water for ten minutes you can be sure that there will be no diseases or bacteria that will harm you. Distillation is for when there are chemicals in the water, or, as you can see from my tests here, the water has minerals dissolved in it that make it undrinkable for humans in any quantity. That includes salt water. A “desalination plant” is just a giant water distillation machine. My drainage pond was originally a brilliant idea for a source of backup water, but there must have been a sulfur deposit exactly where the hole is because it is yellow and disgusting. Supposedly livestock are able to drink that stuff, but it would have to be livestock you really don’t like. It is yucky water and I wouldn’t want to have to drink it as is.

This type of still has a column that turns downward, through a cooling jacket, as opposed to a coil.
The still you see here was one I bought on Ebay for $90, and it is of course made for distilling moonshine. This seller has several sizes, for as little as $55 currently in a 6 quart size (though I suspect they will be sold out by early on Monday when the Digest flies). This is a 3 gallon size here in the pics. The Federal Government does not prohibit the sale of stills to the general public, but they do require that all of the larger still makers give them a list of their customers. I firmly doubt that these guys on Ebay are giving anyone a list, and in general if you Google around you’ll find that there is pretty much no enforcement of the moonshining laws for people who don’t sell the moonshine. Some states have even passed their own moonshine laws allowing small production of alcohol for personal use, and these days you can even get a Federal permit to produce Ethanol. So, to make a short story long, running a still to purify water shouldn’t spook you.
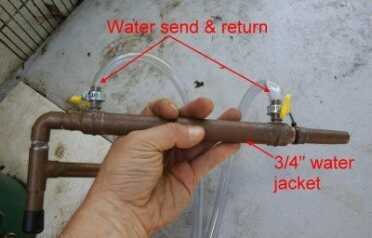
In this type of system you use an outside pump that pumps water through the jacket, cooling the inner pipe and condensing the steam.
The physics of distillation are simple. Boil water in a sealed system with a vent tube at the top. The vent tube passes through some sort of cooling system, either a water cooled jacket, or it winds into a coil that passes through cool water. Steam builds in the pot and eventually it is forced into the vent tube. Then, when the steam passes through the cooling system, it condenses into water, which drips out of the tube, pure and drinkable. The yucky stuff is left in the pot for you to scrape out later.
Of the two types of cooling systems, the one you see here is more complex, but it is very portable and compact. The vent tube is made of 1/2″ copper pipe soldered onto a 3/4″ threaded end, and that threaded end is screwed into a knockout hole cut in the lid of a regular old soup pot. The lid has a ring of silicone around the rim, and it is held tight to the pot by small sized office clippy paper clips. This is not a high pressure system so you don’t need a pressure cooker or canner as your boiling chamber. The silicone and clamps work fine. I was also suprised that his system of just screwing the fitting into the knockout hole with a tightened nut against it was sufficient to hold up the vent pipe without it being floppy.

The water runs to an electric pump. For survival, this type of still is probably not as good as a coil type, but if you have a solar system it certainly doesn’t take that many watts to run the small fish tank pump.
The 1/2″ pipe bends at an elbow and turns down. After that is soldered on a 3/4″ copper jacket, and in that jacket are the water send and return for cooling. This kit actually comes with a fishtank water pump, and I used a separate 6 gallon bucket of water as my send and return water, which has to be changed over time as it heats up from cooling the steam. This system has a thermometer in it which you need for the production of alcohol, but if you are duplicating this design for survival water purification only, it would not be required. You could make this in an hour, if you have the knockout tool and you know how to cut copper pipe and solder.
Copper is also not really required, but you’ll find it in a lot of moonshine stills because there is a reaction between alcohol and copper that is thought to be pleasing to the taste. If you just do a search on Ebay for “moonshine still” you’ll see over 1,400 listings right now, and many of them from China use stainless steel bendy pipe instead of copper, which I suggest if you can afford them.

This is the coil type of still, and this one is currently about $80 on Ebay, without the pump. Note that it has a send and return as well, but you could technically just keep changing the water. I don’t like this design of one pot on the other though. You are heating the water that needs to be cooler.
The other type of cooling system is a coil that winds through a bucket or pot of cooling liquid. In a non-survival situation, most people prefer the coil system and put ice in the bucket, but even for survival the coil system may be better because it doesn’t require a pump. You would have to change the water a lot, and dirty water is fine, but at least you wouldn’t have to worry about reliable electric stored in batteries. I have both types, but this is the only one I have tried so far.
What surprised me was just how much time and fuel it takes to distill any quantity of water. You could make a larger system that would produce more water, but it will also require more fuel. As you can see, I wanted to run this on just my StoveTec Rocket Stove, and it did work, but I had to constantly put more fuel into the fire to maintain a hard boil, and even at that I got all of a half gallon per hour. If I was making moonshine that would be fine, but for water it is kind of lame for he effort. Thankfully with the Rocket Stove, your fuel is made up of sticks that you can pick up off the ground. I got drinkable water, enough to survive, but over months and years I would have to bring fuel from further way, or bring the stove to where the fuel is still easy to find. Absolute survival? Yes. Comfortable survival? No.

Starting with this color water and ending up with drinkable clean water is really amazing, but it takes a good deal of fuel and only produces 1/2 gallon per hour at best.
I also then tried the same system using a propane burner under the pot, just to see if it made more water, but it did not. The propane boiled the pot harder, enough that steam escaped the lid, and enough that the condensation did not work fast enough so some steam came out of the vent tube end. But more usable water in less time not from what I could tell. I had to change the water in the cooling system quicker.
It is pretty nifty to put in dirty water and get clean water out. That much I can say. But from a survival perspective, while it is certainly not a bad idea to have a distillation system available, I would not count on it for long term survival unless you have to. The quantity of water for the effort was really disappointing, and not what you would think at all. I will be working on a new system to get water out of my well instead of dealing with the nasty pond water. If anyone has any valid input to get more out of such a device, please chime in, but don’t start with “You can just…”.








A large pressure cooker works great and it certainly will not implode since as it cools down it simply draws air back up through the cooling coil… I am using one and guarantee it works. No need to mess around with “Office clippy paper clips”!
Now what on Earth is “boneheaded” about suggesting to find a camping (or bug out) site that has a natural spring of cool fresh water? For me that would be the number one priority! A mountain stream seems great until you hike a couple of clicks upstream and find a dead animal floating in your drinking water. Yeah, I know all about filters and boiling, etc., but a mountain makes a pretty large filter, wouldn’t you agree?
“Distillation is for when there are chemicals in the water, or, as you can see from my tests here, the water has minerals dissolved in it that make it undrinkable for humans in any quantity.”
Great idea, but not the whole story. Distillation will remove inorganic ions (metal salts), as well as kill most pathogenic organisms, but it will not remove semi-volatile pollutants like herbicides and pesticides. Higher boiling organic chemicals co-distill in steam.
Distillation is not a replacement for RO systems which use a membrane and filtration at the molecular level. Lithium ions will pass through an RO membrane because they are so small, but not much else.
Water contaminated with organic molecules cannot be purified by distillation. High boiling organic molecules form an azeotrope with water. An azeotrope is a mixture of two or more liquids whose proportions cannot be altered by simple distillation. When an azeotrope is boiled, the vapor has the same proportions of constituents as the unboiled mixture.
Or else I wasted all those years of chemical education and the time I spent working for EPA.
They would have lower boiling points than water, which has already been mentioned here. RO systems waste up to 90% of their water into the waste pipe, and they require pressure. As discussed in the water article here, RO is not practical for a survival situation without significant backup.
Will activated charcoal, as a final filter, reduce the amounts of “Higher boiling organic chemicals” or VOCs in the final product?
Something I can actually use and well written too. I like most of the articles but this one hit home. Years ago I obtained a gasohol permit and built a beautiful little still, all polished steel. copper and glass and it was due to a pressure recirculating system that used Jack Daniels re caromed barrels and their charcoal produced very drinkable Bourbon in under a year. I never sold it but gave it away. My wife destroyed the still when she found my daughter selling uncut whiskey for $1.25 a gallon. Now id the time to start over and if good drinking water comes from it all the better.
Bottom line SHF scenario, those in the city die. Once the resources are gone or claimed, city dwellers will start to die off from disease, fellow humans, or lack of resources such as clean water. The mountain men were survivalists. Go to the mountains, drink spring or well water after boiling it if possible. Hide from the big gangs and wait for civilization to come to some form of order. Don’t keep a bunch of stuff everyone will want to kill you for. Learn to live off the land with little to no resources. More stuff means bigger footprint means bigger target. As for water distilling, better find a well, stream, or stick to dew.
So lets take a poll. Should we delete bonehead useless comments like this or should we allow them?
Just because I don’t agree with distilling water does not make the comment bone headed. If you research making safe distilled water you would know that the thermometer you pooh poor is necessary. If you boil too fast you carryover bad water into the “good” water you are making. That will happen if you force a fast boil like the gas burner. If you collect before you get the temp to 212 f you get chemicals with low boil points in the “good” water. So why not just boil what you can find if you aren’t going to do the research and do it right. I was in the Navy and we distilled water every day. I’m not without some knowledge about it. Even with our systems, we dumped more water from our distiller than we kept because it was no good.
Very well said, to add to comments the distillation head on that pot still is a piece of (crap) junk. I think you were taken on that purchase.
A pressure cooker would probably be a better choice for the still. Easier to plumb and no loss of steam due to the seal. As has already been mentioned, you better have an idea of the possible contaminates. Keep in mind the purpose of a “moonshine still” is to remove the alcohol from the water, with the water being the waste product. To me the best use of a still would be for good clean salt water (if you are lucky enough to be near the ocean).
Wrong. But thanks for the useless advice based on nothing. The problem with sealed vessels is that if for some reason the vent gets plugged and the pot cools down, the vacuum created will implode your system at its weakest point. There is no need for a pressure system in a low pressure environment, and it would be needless cost and weight regardless.
Great article and comments. I am a former Air Force Survival Instructor. The comment on using a solar still is a good one. The Air Force used to pack one in many survival kits. Sorry but I have to disagree with your observation on pressure cookers. I spend several years in Saudi Arabia teaching Desert, Mountain, and Water survival to the RSAF. We used 5 gallon pressure cookers as stills to make Sid, the native alcoholic beverage. Using 3/8 ID copper tubing for the coil you never get into a high pressure situation. Not saying that you have to use one or that it is the best way. But there were literally thousands of these in country with never a problem. Aramco used to give an instruction sheet to every new employee, on how to use one to make Moonshine. You are not going to get a vacuum strong enough to implode a pressure cooker. We have used them in lab experiments as a vacuum chamber. Mash is much thicker then any water source, and we poured it straight into the still. Just use at least 3/8 ID copper tubing for your coil, the pressure will keep it clear. I had two and never had a problem, while making hundreds of gallons of shine. At $100 US for 5 gallons at 160 proof, it was worth the bother.The thermal mass of the pressure cooker will make it easier to maintain temp.I agree that in a mobile survival situation that the weight of the pressure cooker would be a unnecessary hindrance.
You could preheat the water: A coil of black plastic pipe in a box covered with glass on a sunny day. Also do NOT use lead solder if you build one use silver solder or compression fittings.
So was the reason it was slow was the process just slow by nature, or is the stove ineffeciant for what you are doing or was the setup to big for the stove? Just curious as to what you think really bottom line on it. I am actually going to try this this summer. We have a creek that runs through our property. I might build my own unpowered distilling machine. This was a great article by the way. Never even thought about distilling as a way to get drinking water. I was going the purify route of charcoal sand and all that jazz but was going to buy a steri pen and do a nice combo but with the distiller you get perfectly safe drinking water. Thank you.
I think that more square inches of condenser is the answer for better performance. For a stream condenser you could get a huge coil. I bought one of these:
http://www.ebay.com/itm/moonshine-still-condenser-stainless-steel-Popcorn-special-Heat-Exchanger-/331492713557?
but the guy doesn’t seem to be selling them anymore.
So larger coil to condense the steam. Use a pot that fits your burner not so huge that it will take a week to bring it to a boil but not so small that it evaporates before it has a chance to steam larger coil with some nice cold water.
While this still set up is nice for making moonshine, it is a poor, to very poor choice for using as a water still. Most of the reasons have been listed.
1. The most serious one period, it traps everything that evaporates and tends to condense it in the coils. Very, very bad if you have an unknown, very low grade source! For reasons already mentioned, it will not allow many hydrocarbons to separate from the water! If you have alcohols or even gasoline or diesel contamination, it will foul the water that comes out.
2. Uses too much fuel!! While there are some way around this, like using available wood sources, old tires, etc… it can and would be a problem in most survival situations. Not to mention it would give a huge signature of your position to others, not always a good idea.
If one wants to create a lot of water cheaply, efficiently and with few resources being used try a solar still.
They have their drawbacks, every system does, but fewer than this disaster looking to happen.
This is one example of an effective solar still and it is less likely to kill you with contaminants over all. http://www.offthegridnews.com/grid-threats/how-to-build-a-solar-powered-still-to-purify-drinking-water/
I have seen still similar to this used to create safe drinkable water at the rate of 3 gallons a day in the south during the summer and even around 1+ gallons a day up north on a kind of sunny day.
Google solar stills, there are a lot of plans out there, most work pretty well and give water up in manners most would not think possible.
Best part is low to no fuel costs and some have almost no material cost, many are portable.
Any are a better choice than this monstrosity!
I was going to suggest the same thing. I made one very similar to the one in the link for the science fair when I was in 5th grade in the mid 1960’s. I also made a solar furnace with a Fresnel lens, and a folding solar “oven”. Inexplicably, I lost top prize to a girl who spit into a petri dish and grew bacterial cultures. I think I was ahead of my time.
If you are going to make your own still or repair one please use pure tin solder with NO lead in it. Leaded solder will lead to lead poisoning (with out the holes in you).
IF you are going to solder up your own parts ABSOLUTELY
You might want to re-think your “nut” on the copper fitting. That is a “locknut” used in the electrical trade to fasten conduit to boxes. They are generally cadmium-plated or galvanized. Eventually, a galvanic corrosive action will take place between the locknut and the copper fitting and cause a leak, or worse, possibly contaminate the water. You should use brass, copper, or stainless steel.
FYI. Those “Office clippy paper clips” are known as “Binder clips”……you can find them in office supply, art stores and probably even drug stores now?
If you have a well you can use a sand bucket. It would require removing the pump and lines but is a very viable source. Lehmans Hardware is an excellent source for such items.
You could buy a new radiator and plumb it into the system ,then it would handle all you could make!
You shouldn’t use a radiator for drinking water. There is zinc and often even lead in the coatings.
Zonks! That’s a lot of work and equipment just to distill water.
In the camping industry one can buy the easy to use and setup water distiller that fits on the camp fire rack. Just fill the basin and enjoy clean fresh distilled water in 20-mins. As for your pond water to try or to keep the camping distiller easier to clean, pour the pond water through a coffee filter first.
The other option is to use the gravity water feed Berkey system. There are plenty of Youtube vids of pond water, ditch water and rain water with this gravity system. Even to make your own with 2 Berkey filters and 2X 5-gallon buckets on the cheap.
When bugging out/in and there is no electric for running the pumps, natural steam and gravity systems win hands down. You are on the right path, just a little shy hitting the target.
Now, if you are on the cheap (strapped for cash) and have a simple teapot, one can make a great water distiller like this one.
Homemade Water Distiller – DIY – Stove Top or Camping Stove Top “Pure Water” Still – EASY instructions!
https://www.youtube.com/watch?v=00kKPOs_FA4
It’s still good to raise awareness of prepping for good clean water. Cooking, drinking, bathing are required to keeping healthy in any disaster situation. Glad you took the time getting it out there.
A thought on water from your well, take a 4 foot long piece of thin wall 4 inch pipe glue in a 4 to 3 inch reducer, place a bail (metal or wire support on other end), then put a trimmed new toilet plunger handle side down and trimmed to about 3 inches length into bottom of the pipe, secure loosely with a metal strip on outside of pipe into the wooden handle. The idea is that as you drop the unit into the well the rubber plunger head floats up an inch or 2 from pipe weight, water rushes into pipe lift your pipe out via your rope the plunger head seals the reducer end of pipe and you pull up your water. For ease of operation it is best if your submersible/piping have been previously removed so the water pail (pipe) goes up and down easier, cost $20 and 20 minutes
When my wife and I brought property in, a very rural area, (no county water) she did research on old fashioned hand pumps and when the well was put in we installed a Bison stainless steel hand pump that sits on the well head.
If we lose power we will still have acces to water for cooking, flushing toilets, and bathing.
Bison’s are not cheap and I hope we will never have to rely on for an extended period if electricity fails but I’m glad we made the investment.
Great article.
IF boiling the water faster only causes the extra steam to escape around the edges due to additional pressure, then add one or even two extra copper out put lines into your evaporator. That should compensate for the extra pressure and give you more water condensation from your cooler. just a thought.
Please keep in mind, fuesal oils (poisonous), alcohols, aldehols, all have lower boiling points than water. If you will allow all of the “steam” to vent until you get to 212F your “pure water” will be much better for you. Otherwise, you didn’t remove any of these products with lower boiling points. Also, some nasty stuff has higher boiling points, so keeping the temperature at 212F or just barely over is even more important so more “stuff” is left behind.
Yes, I agree too. Even some bacteria and viruses will not die nor their protective barriers breached below the 212F or 100C minimal temp. The so called rolling boil works great.
The real question is. How much sulfur do you get out of it?
Not enough to make a firecracker lol.
Plumbed into the family fireplace a system like this could run as long as heating and cooking were going.
Would an increased length of coil produce more water when introducing more calories at the primary end? Multiple coils/outlets?
You would think that more and larger the coils, the more BTUs you could utilize to boil the water faster. In practice it may not work that way.
More coil would definitely provide more condensation. The trade of is in cooling the coil.
The answer is no. The amount of steam produced is limited by the surface area of the water. So to get more production you need a larger surface area of the pan. Water boils at 212 degrees and any heat you add above that required to maintain that temp is largely wasted. The coil length is irrelevant so long as you are not getting steam exiting at the finished product end. The advantage of a longer coil would be a lessor need for a cooling tank or at least one that was not as cold would still do the trick.
Find some way to induce a slight vacuum on your boiler. water boils at much lower temperatures at lower barometric pressures.
At a bomplete vacuum your water would turn to vapor at ambient temps.
And bacteria are more likely to survive that lower temp, hence, the boiling part, the vapor is working as a filter and I would not trust it 100% without the heat.
Put dirty water in a container like a washtub. Put a smaller empty container, like an empty coffee can, in the center (you may have to weight it to keep it from floating) cover the washtub with semi opaque plastic sheeting. Put a rock on the plastic directly above the coffee can. Put the whole shebang outside. The sun will create greenhouse effect and evaporate the water. The water will condense on the plastic and run down the depression caused by the rock and drip into the coffee can.
On a sunny day this will get you a gallon or two of clean distilled water a day. It also works on cloudy days but will not distill as much per day. The water evaporates leaving dirt and germs behind. This is called a solar still!